Pitfalls in the Diagnosis of Dysplasia
The pathologist must be aware of situations in which reactive epithelial alterations may mimic dysplasia in order to avoid its over-diagnosis.
Ulcer/Erosion with marked active inflammation
Active inflammation may cause nuclear changes that mimic high-grade dysplasia. One should be cautious when numerous neutrophils infiltrate the overlying epithelium; however, the degree of inflammation is important to note. Barrett’s esophagus with high-grade dysplasia may be associated with a few neutrophils in the overlying epithelium, but the nuclear changes are so dramatic that the diagnosis of high-grade dysplasia can be made. If numerous neutrophils or an adjacent ulcer are present, we avoid the diagnosis of dysplasia and use the category of "indefinite for dysplasia." Chronic inflammatory cells do not cause the nuclear abnormalities associated with active inflammation.
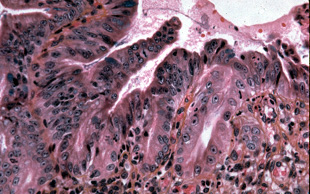
This biopsy is from an ulcer edge. It displays changes mimicking high-grade dysplasia.
Metaplastic columnar epithelium underlying squamous epithelium
Use of proton-pump inhibitors may cause caudad migration of the squamo-columnar junction, or squamous islands may develop within the Barrett’s segment. In either case, Barrett’s metaplastic epithelium may still present underneath the squamous epithelium on the surface (squamous overgrowth). Because dysplasia and adenocarcinoma may develop within the metaplastic columnar epithelium underneath the squamous surface, the endoscopist must biopsy these squamous islands. Unfortunately, since no overlying columnar epithelium is present for evaluation (being completely replaced by squamous epithelium) the criterion of mucosal surface involvement by atypical epithelium cannot be used to make the diagnosis of dysplasia. We look for marked nuclear atypia in combination with architectural distortion before raising the possibility of dysplasia. We are hesitant to make the diagnosis of low-grade dysplasia based on the deep glands; but we think that a diagnosis of high-grade dysplasia can be rendered if the nuclear features (loss of nuclear polarity, hyperchromatism, pleomorphism) are consistent with high-grade dysplasia.
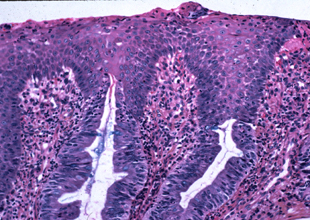
Metaplastic columnar epithelium adjacent to a squamous island
Frequently the columnar epithelium immediately adjacent (1 to 2 glands) to a squamous island displays atypical nuclear features that are present on the mucosal surface. We are hesitant to make the diagnosis of dysplasia in this context. We like to see the nuclear atypia involve the surface epithelium in areas several crypts away from the squamous island before making the diagnosis of dysplasia. The questions arises, "How far is far enough?" We believe that in Barrett’s esophagus negative for dysplasia, the epithelium on the mucosal surface should appear normal 2 or 3 glands away from the squamous island. Abnormal nuclear changes beyond this point probably indicate dysplasia.
Detached fragments of atypical epithelium
The surface epithelium in Barrett’s esophagus may become detached from the lamina propria, and these detached fragments may display features suggesting dysplasia. For reasons that are not clear, but possibly due to the trauma that removed them from the surface, the nuclei of the cells within these strips of detached atypical epithelium may appear highly atypical when there is no dysplasia. We hesitate to make the diagnosis of dysplasia based on these fragments if no dysplastic epithelium is present within the intact biopsy. Almost as a rule, we do not diagnosis high-grade dysplasia based on detached fragments alone. If the nuclear changes are highly atypical, we would consider the diagnosis of indefinite for dysplasia or, possibly, dysplasia, without assigning a grade.
Reactive gastric cardiac mucosa
Reactive gastric cardiac mucosa displays nuclear atypia that may be misinterpreted as dysplastic metaplastic epithelium. The absence of goblet cells may be an important clue; but it must be remembered that in dysplastic epithelium, goblet cells may not be seen on biopsy specimens. Dysplastic epithelium lacking goblet cells will display greater nuclear atypia than seen in reactive gastric cardiac epithelium.
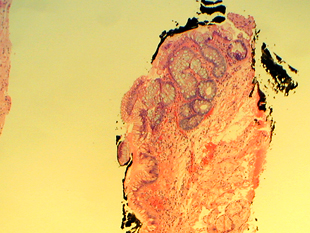
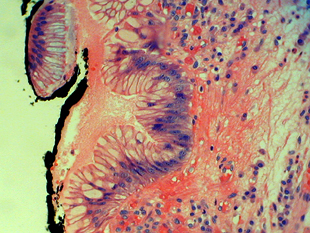
Metaplastic columnar epithelium is present adjacent to gastric cardiac mucosa.
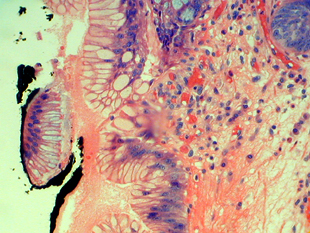
The gastric cardiac mucosa from the case above demonstrates reactive nuclear atypia that may be misinterpreted as dysplasia.
Pseudogoblet cells in the gastric mucosa may be contribute to the misinterpretation of dysplasia in this case.